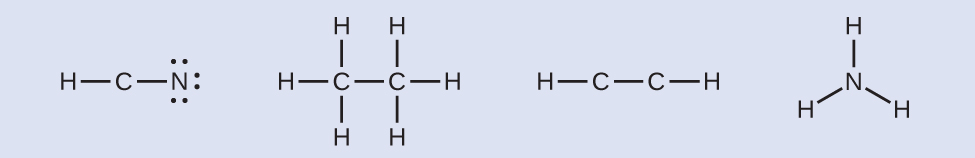
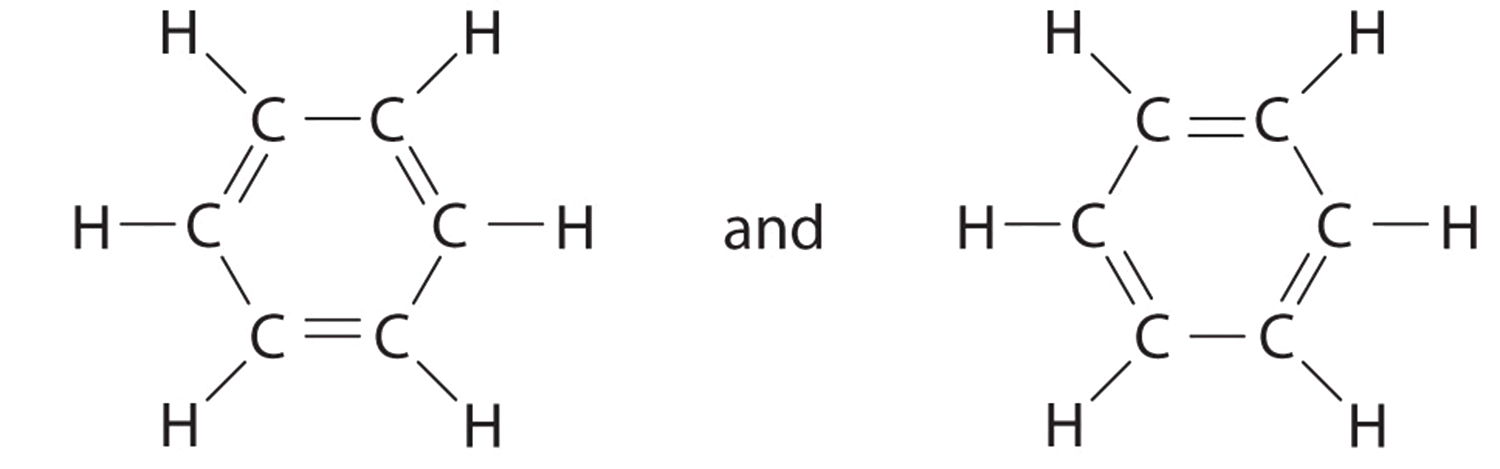Basic Concepts of Chemical Bonding Chapter 8. Three Types of Chemical Bonds Ionic bond Ionic bond –Transfer of electrons –Between metal and nonmetal ions. - ppt download
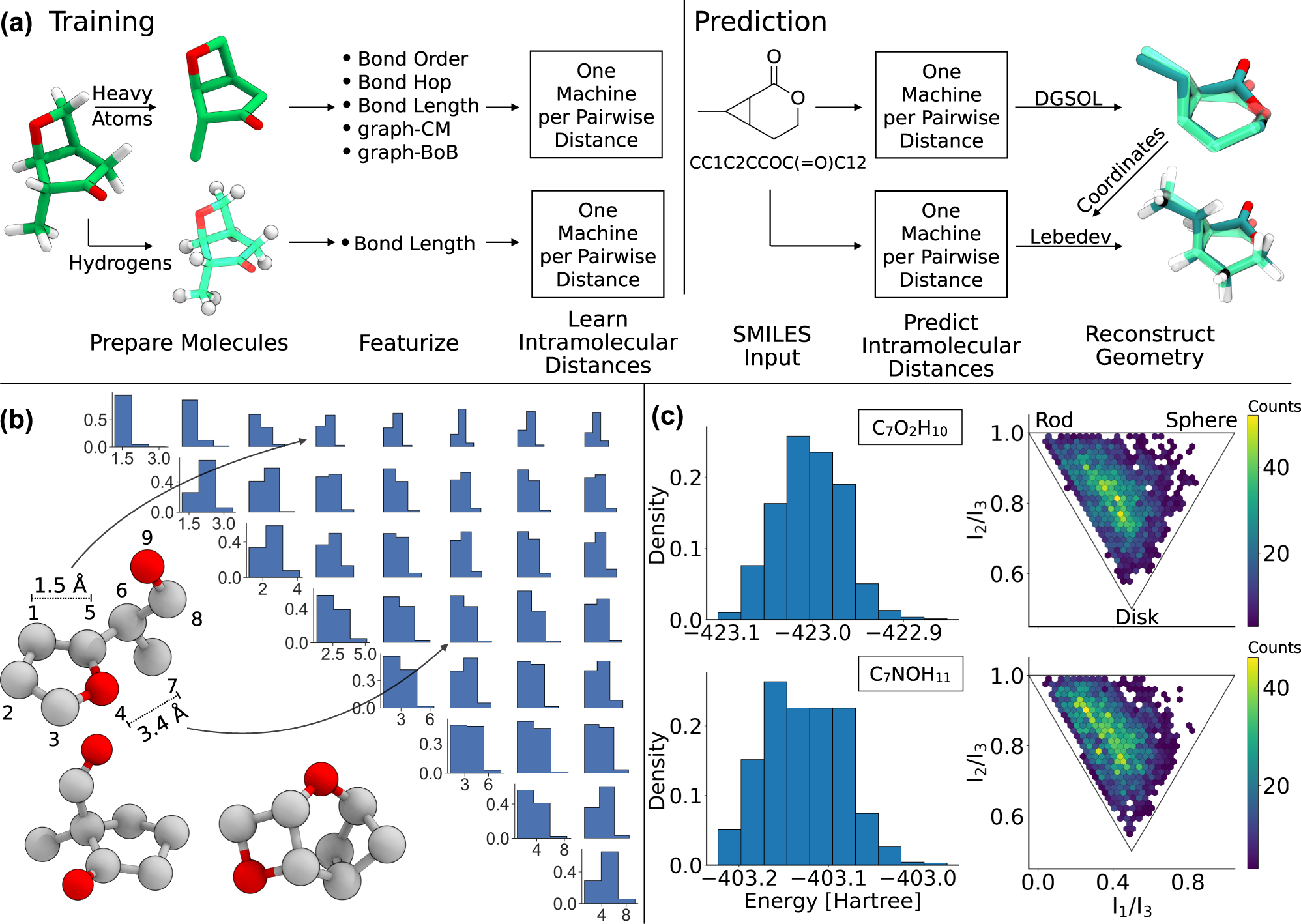
Machine learning based energy-free structure predictions of molecules, transition states, and solids | Nature Communications

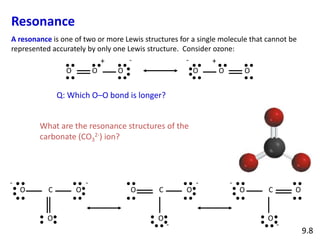
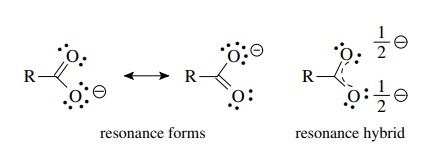
Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Explain why CO_{3}^{2-} ion cannot be represented by a single Lewis structure. How can it be best represented?

All questions go with Model 4 , please label thank you in the Ltwis suettre for PCls and XeFe Model 4: Which Lewis Structure is Better? The two structures below could represent

Explain why CO_(3^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?






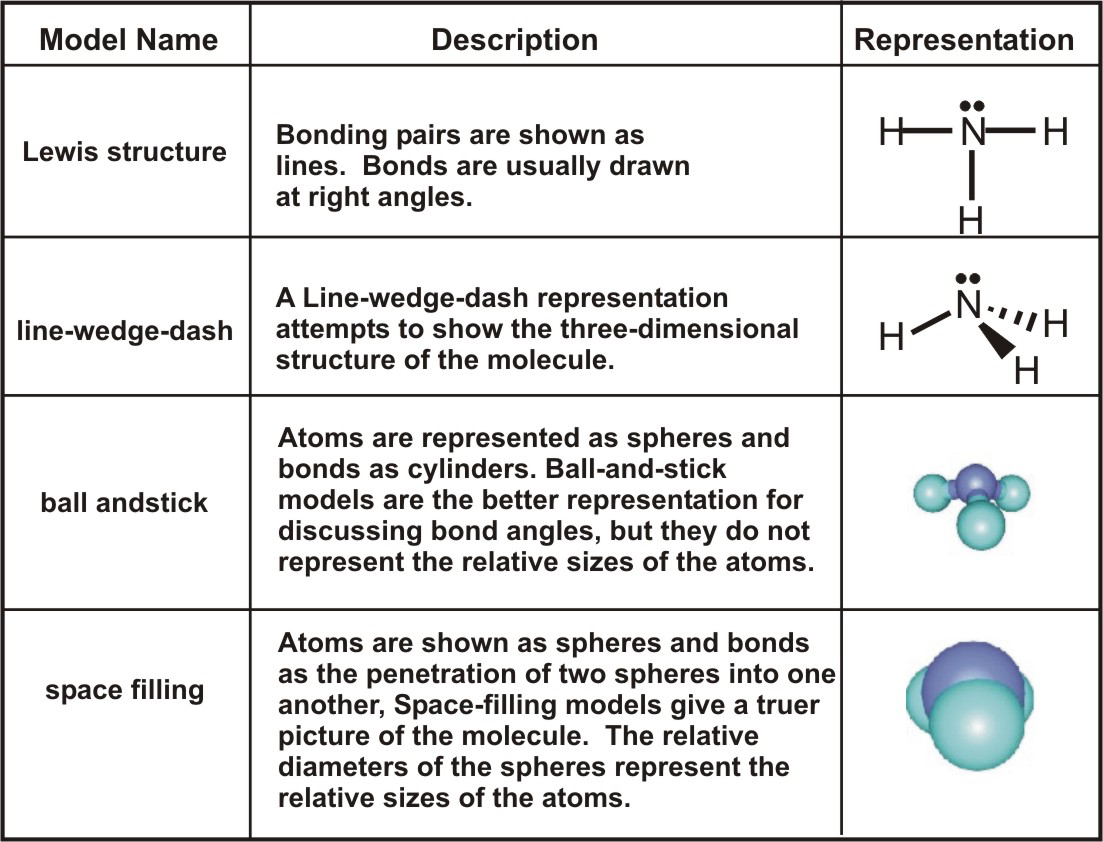

/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)